Navigating Medical Cannabis and the Endocannabinoid System
History of Cannabis
In written history, the cultivation and use of the plant dates back at least the third millennium BC and possibly as far back as the Pre-Pottery Neolithic B (8800–6500 BCE). Cannabis was farmed for fibre, food, medicine, and religious/recreational purposes, with evidence from Neolithic times and ancient China/India, used by Scythians and Hindu saints. Its medicinal use grew in the 19th-century West, while later 20th-century global restrictions emerged, only to see recent movements toward decriminalisation, showcasing a long, evolving history.
Source: Dr Russo, E.B. “History of Cannabis and Its Preparations in Saga, Science, and Sobriquet”, 2007.

Known medical uses
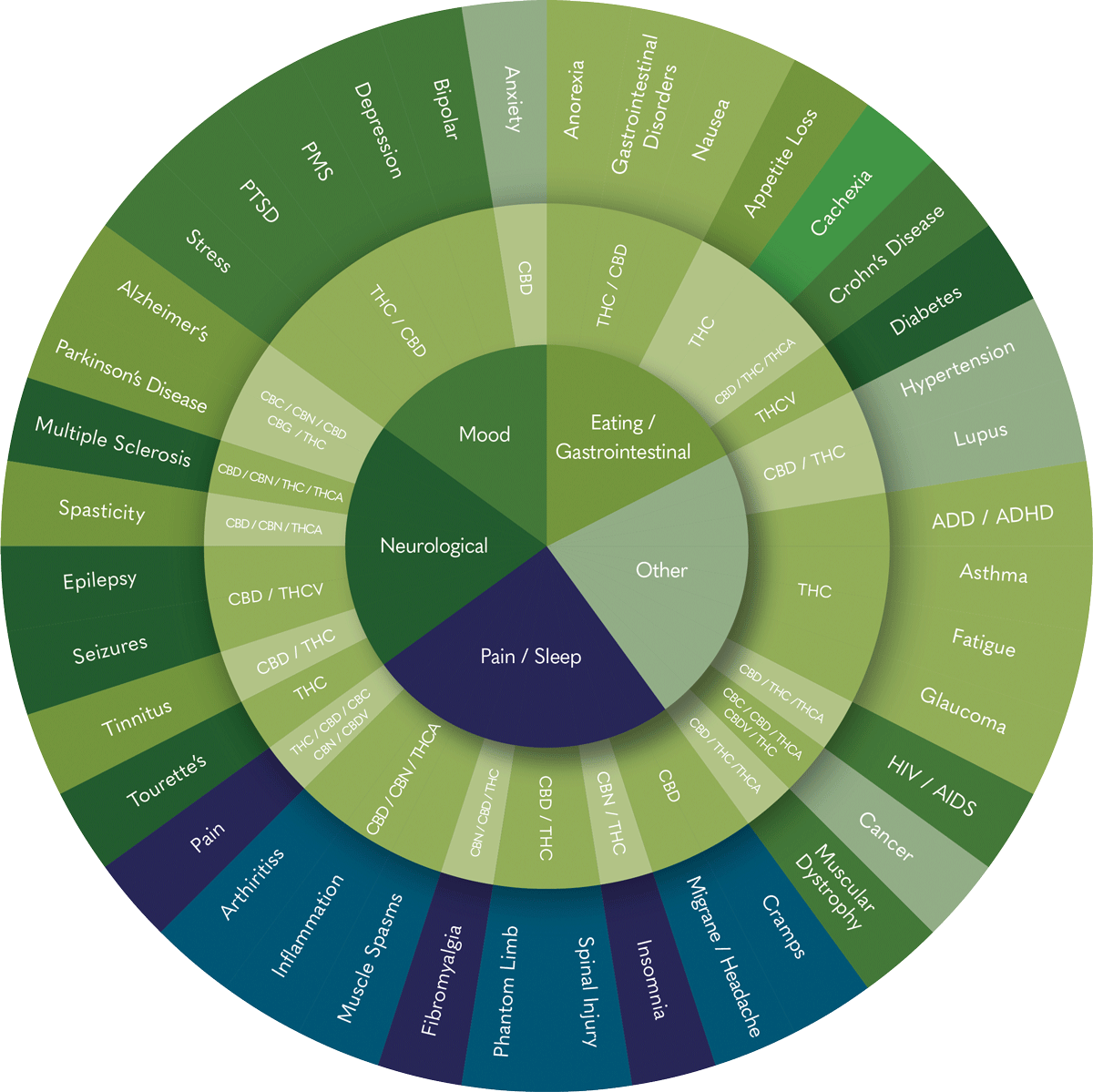
Phytocannabinoids act on the body's universal regulatory system (ECS). Consequently, the medical industry has investigated phytocannabinoids potential for a diverse range of conditions. Based on data from the German BfArM Companion Survey (2022) and international scientific reviews (Health Canada, 2018), common areas of application have included:
-
Conditions and symptoms that fall into this category include:
Chronic Pain, Arthritis, Inflammation, MusclesSpasms, Fibromyalgia, Phantom Limb, Spinal Injury, Insomnia, Migraine/Headache and Cramps -
Conditions and symptoms that fall into this category include:
Alzheimer’s, Parkinson’s Disease, Multiple Sclerosis, Spasticity, Epilepsy, Seizures, Tinnitus and Tourette’s.
-
Conditions and symptoms that fall into this category include:
Anxiety, Bipolar, Depression, PMS, PTSD and Stress.
-
Conditions and symptoms that fall into this category include:
Anorexia, Gastrointestinal Disorders, Nausea, Appetite Loss, Cachexia, Crohn’s Disease and Diabetes.
-
Conditions and symptoms that fall into this category include:
Hypertension, Lupus, ADD / ADHD, Asthma, Fatigue, Glaucoma, HIV / AIDS, Cancer and Muscular Dystrophy.
Sources: BfArM, “Abschlussbericht Begleiterhebung”, 2022); Health Canada, “Information for Health Care Professionals”, 2018); NASEM, “The Health Effects of Cannabis and Cannabinoids”, 2017).

When is cannabis prescribed?
Therapeutic Goals & Potential Benefits
Medical cannabis is primarily prescribed as a therapy for severe, chronic conditions where standard treatments have been exhausted or poorly tolerated. Based on the current DGS Practice Guidelines (2024) and data from the BfArM (Federal Institute for Drugs and Medical Devices) companion survey, the therapy focuses on the following clinical goals:
Modulation of Chronic Pain
Target
Primarily neuropathic (nerve) pain.
The Benefit
The goal is a relevant reduction in pain intensity ("pain relief") rather than total pain freedom. By modulating pain perception in the central nervous system, patients often report an improved ability to cope with persistent symptoms.
Relief of Spasticity
Target
Multiple Sclerosis (MS) and other neurological conditions.
The Benefit
Cannabinoids (specifically THC/CBD combinations) have been shown to reduce muscle stiffness and spasms. This can lead to improved mobility and a reduction in associated pain for patients who do not respond to standard antispastic medications.
Symptom Control in
Palliative Care
Target
Oncology and severe chronic illness.
The Benefit
Used as a supportive therapy to manage the side effects of severe treatments (e.g., Chemotherapy).
Nausea
Reduction of chemotherapy-induced nausea and vomiting (CINV).
Appetite
Stimulation of appetite in cases of cachexia (wasting syndrome).
Improvement of Quality of Life
Target
Secondary symptoms of chronic illness.
The Benefit
Chronic illness often disrupts sleep and mental well-being. A key benefit observed in German accompanying research is the improvement of sleep quality and general well-being ("Quality of Life"), often resulting from the sedative and relaxing properties of specific cannabis varieties.
SOURCES:
DGS Praxisleitlinien, “Praxisleitlinie: Cannabisbasierte Medikamente in der Schmerzmedizin (Version 2.0)” 2024
BfArM, “Abschlussbericht der Begleiterhebung nach § 31 Absatz 6 des Fünften Buches Sozialgesetzbuch zur Verschreibung und Anwendung von Cannabisarzneimitteln”, 2022. (Download Link)
The Endocannabinoid System (ECS)
The endocannabinoid system (ECS) is a widely distributed signalling system in the human body that is involved in the modulation of numerous physiological processes. One of its central roles is the regulation of homeostasis—the dynamic balance of internal processes—alongside functions related to pain perception, immune signalling, metabolism, stress responses, and neural activity.
The system consists of three core components:
Endocannabinoids - the bodies natural messengers:
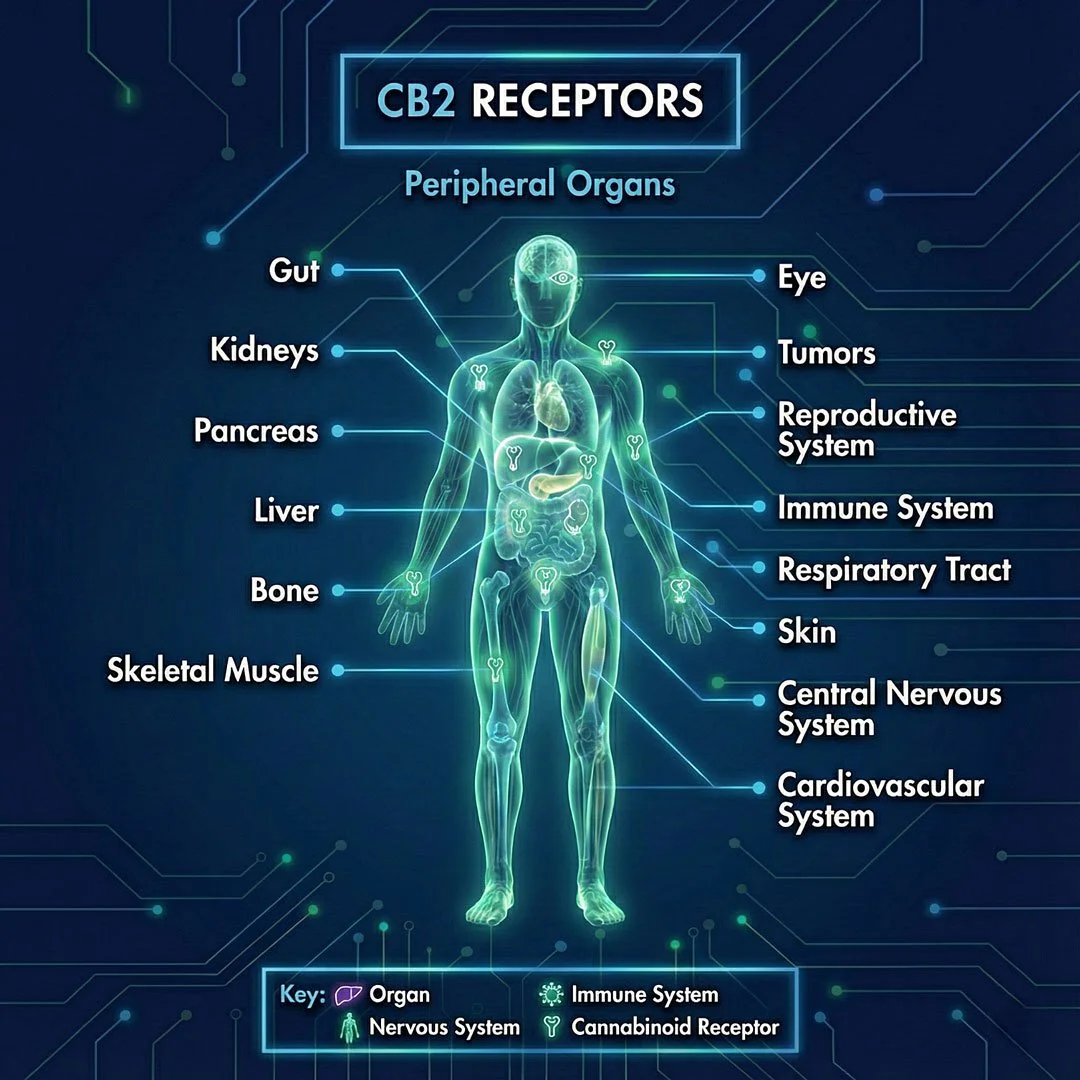
Lipid-based signalling molecules produced endogenously by the body, including but not limited to well-characterised compounds such as anandamide (AEA) and 2-arachidonoylglycerol (2-AG).Receptors (CB1 and CB2) - the bodies mailboxes:
G-protein-coupled receptors expressed across the central and peripheral nervous system, immune cells, and various peripheral tissues, where they mediate and modulate cellular signalling.Enzymes: Enzymatic pathways responsible for the synthesis and degradation of endocannabinoids, regulating the intensity and duration of endocannabinoid signalling once these molecules have fulfilled their function.
How it works: A helpful way to understand the endocannabinoid system is the lock-and-key analogy. Cannabinoid receptors can be thought of as “locks” on the surface of cells, while endocannabinoids act like “keys” that bind to these receptors and trigger signalling processes.
In situations such as pain, inflammation, or stress, this signalling helps the body adjust and regulate physiological processes. Rather than acting as a simple on/off switch, the endocannabinoid system supports fine-tuning and balance within the body.
Source: Pertwee RG (Ed.). Handbook of Cannabis. Oxford University Press, 2014.
What do Cannabinoid (CB1 & CB2) receptors do?

Phytocannabinoids – Nature’s Keys
The cannabis plant produces over 100 bioactive compounds known as phytocannabinoids.
Some of these plant-derived compounds share structural and functional similarities with the body’s own endocannabinoids, enabling them to interact with and modulate the endocannabinoid system (ECS).“
THC
(Δ9-Tetrahydrocannabinol):
THC is the primary psychoactive phytocannabinoid. In a medical context, it is used for its central nervous system effects, including muscle relaxation, modulation of pain perception, and appetite stimulation.
CBD (Cannabidiol):
CBD is a non-intoxicating phytocannabinoid. It is actively researched for its modulatory effects on inflammatory signalling, stress-related processes, and muscle tone, without producing the characteristic intoxicating effects associated with THC.
The "Entourage Effect" (Phytocomplexity)
Medical cannabis contains more than just THC and CBD. It also includes other compound classes such as terpenes (aroma-active constituents) and flavonoids.
The concept commonly referred to as the “Entourage Effect” describes the hypothesis that multiple plant compounds may interact to influence overall effects and tolerability. While this concept is still under scientific discussion, it is often used to explain why full-spectrum preparations can differ in their subjective and clinical profiles compared to isolated substances.
How is treatment prescribed?
Since April 1, 2024, the legal framework for medical cannabis in Germany has changed. With the removal of medical cannabis from the Narcotics Act (BtMG), it is now prescribed on a standard prescription (Rx) where medically indicated.
This means that a strict “last resort” requirement is no longer a formal prerequisite for issuing a prescription in every case. However, for statutory health insurance (GKV) reimbursement, specific criteria and a prior approval process may still apply..
Use this 4-step guide to prepare for your consultation.
Stage One: Documentation Preparation
Even though cannabis is no longer prescribed under BtMG rules, your doctor will still need a clear medical rationale. Good documentation supports a well-informed decision.
Compiling "Therapy History"
To documenting your condition clearly, consider the following:
Diagnosis List: Relevant medical findings confirming your condition (e.g., chronic pain, MS, migraine).
Current Status: A short symptom log and how symptoms affect your daily life.
Prior Treatments: A list of medications and non-drug therapies you have tried (incl. outcomes and side effects).
Why this helps: Even if not strictly required for a private prescription, structured documentation helps your doctor assess whether cannabinoid therapy is appropriate and proportionate for your individual situation.
Stage Two: The Consultation
Finding the right physician is key. Patients do not need a “special cannabis doctor,” but a licensed physician who is willing to consider cannabinoid therapy as part of an individualized treatment approach.
The Doctor-Patient Dialogue
Who can prescribe:
Any licensed physician in Germany (except dentists and veterinarians) may prescribe medical cannabis on a standard prescription, provided a medical indication is present.The Current Standard:
Cannabis does not have to be prescribed only after all other options have been exhausted. If your physician considers cannabinoid therapy medically appropriate—for example due to insufficient efficacy or tolerability of other treatments—it may be prescribed directly.How to address the topic:
You may frame the discussion in a neutral, non-demanding way, for example:
“I am interested in treatment options that focus on symptom relief and tolerability. Given the current regulations, could cannabinoid-based therapy be a reasonable option in my case?”
Stage Three: The Reimbursement Application
If you would like statutory health insurance (GKV) to cover the costs of cannabinoid therapy, a formal reimbursement application is still required. This process is carried out jointly by you and your physician.
3. The "Kostenübernahme" Process
The doctor’s role:
Your physician prepares the medical justification (ärztliche Begründung), outlining the indication, disease severity, and why cannabinoid therapy is considered appropriate (e.g., insufficient efficacy or tolerability of other treatments).The patient’s role:
You submit the completed application documents to your statutory health insurer (Krankenkasse). This includes the physician’s medical reasoning and any required forms.Assessment criteria:
For reimbursement approval, the legal framework (§ 31 para. 6 SGB V) generally requires that a serious condition is present and that standard therapies are not available, not suitable, or not sufficiently effective in the individual case.Timeline:
Once a complete application has been submitted, statutory health insurers are legally required to issue a decision within three weeks, or five weeks if the Medical Service is consulted.
Point Four: Prescription Fulfilment
Once your prescription has been issued, it can be filled at any pharmacy (Apotheke).
Redeeming Your Prescription
Where to fill the prescription:
Medical cannabis prescriptions can be redeemed at any german pharmacy. However, not every pharmacy stocks cannabis-based medicines on site, so ordering may be required.Availability and ordering:
If the prescribed product is not immediately available, the pharmacy can usually order it from a licensed distributor. Delivery times may vary depending on product availability and supply logistics.Counselling at the pharmacy:
Pharmacists provide information on correct use, dosage instructions as prescribed by your physician, and practical aspects such as storage and handling.

Important Legal Note for Patients
The content provided on this website is intended for general informational purposes only. It does not constitute medical advice and is not a substitute for consultation with a licensed physician or other qualified healthcare professional.
This website does not promote self-diagnosis or self-medication. Decisions regarding medical treatment, including the prescription of cannabis-based medicines, are made exclusively by licensed healthcare professionals based on individual medical assessment.
Medical cannabis is subject to national regulations and may only be prescribed where medically indicated and in accordance with applicable laws.
While we strive to keep the information on this website accurate and up to date, medical knowledge and legal frameworks may change over time.
For further reading and the sources cited for the information in this website, please refer to our References & Further Reading page.